Electrophysiology Ablation Market 2022
Electrophysiology Ablation Market is expected to reach US$ 6.3 Bn by the end of 2031, with sales revenue registering a CAGR of 8.4%.Increase in worldwide prevalence of AFib with significant effects on associated morbidity and mortality, changing competitive landscape for electrophysiological products, and shifting trend of radiotherapy for electrophysiology ablation are key market trends.
Get Free Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/25474
According to a recent study by Persistence Market Research, the global electrophysiology ablation market is expected to witness high growth from US$ 2.5 Bn in 2021 to US$ 6.3 Bn by 2031. This reflects a CAGR of around 8.4% over the forecast period (2021-2031).
Rising morbidity and mortality due to cardiac disorders, especially arrhythmia, make this market more demanding. Moreover, new product innovation and increased adoption of electrophysiology ablation devices by cardiologists in hospitals is expected to boost market growth over the forecast period.
The market is experiencing drastic changes with high adoption of electrophysiology ablation procedures due to rising awareness about cardiac health. Sales and marketing practices by some manufacturers are influencing the procedure and device cost in selective regions. Additionally, rising investments in the healthcare sector in emerging regions and reimbursement policies for cardiac ablation are further expected to give positive growth to this market.
Technological innovations are warranted to improve the safety and effectiveness of ablation devices, thereby helping market growth. Significant growth has been observed in two of the most dynamic segments: cardiac rhythm management (CRM) and electrophysiology (EP) segment, in recent years.
From the last few years, there has been considerable progress and innovation made in developing advances in heart rhythm therapy. Ongoing researches in this segment will help create the landscape for further product advancement in the electrophysiological ablation market. As the market is consolidated in terms of catheters being used, market leaders are focusing on increasing their product portfolios to strengthen their product acceptability at the end user level globally.
- For instance, in June 2021, Medtronic plc received U.S. Food and Drug Administration (FDA) approval for the Arctic Front™ set of cardiac cryoablation catheters.
Leading players are focusing on investment in developing countries to increase their foothold in the market. Also, major market players have been concentrating on enhancing gains through advancements in distribution channels.
Company Profiles:
- Biomerics
- Biosense Webster
- Abbott Laboratories
- MEDTRONIC PLC
- Boston Scientific Corporation
- CathRx Ltd
- Biotronik SE & Co. KG.
- Japan Lifeline Co
- ATRICURE, INC.
- Auris Health
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/25474
Key Takeaways from Market Study
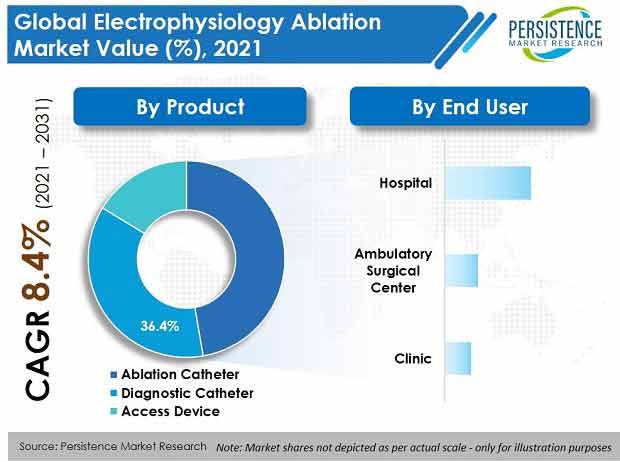
- With advanced product features and more acceptance by end users, ablation catheters hold a high market share around 47.4%.
- 52.5% market share is held by the atrial fibrillation (AF) segment of electrophysiology ablation, due to high prevalence of this disease and need for its treatment.
- Hospitals are expected to enjoy more than half of the market share during the forecast period because of high adoption of electrophysiology ablation devices and procedures and increased number of walk-in patients.
- North America market is expected to grow 2.5X over the forecast duration due to rising prevalence of conditions such as atrial fibrillation and tachycardia, growing awareness regarding cardiac health, and presence of reimbursement policies in the region.
“Increasing adoption of technologically advanced devices and growing awareness about cardiac disorder and associated risks are expected to drive demand for electrophysiology ablation over the decade,” says a Persistence Market Research Analyst.
Who is Winning?
Major manufacturers of electrophysiology ablation devices have been focusing on product launches, collaborations, and partnerships for global expansion.
- BIOTRONIK’s product the Force Sensing Ablation Catheter, the first and only gold-tipped to support effective ablations in the left atrium, got CE approval in 2020.
- Likewise, AtriCure got into a strategic collaboration with Baheal Group for a distribution agreement to market its products across China.
Major market players covered in the report include Biosense Webster (Johnson & Johnson), Abbott Laboratories, MEDTRONIC PLC, Boston Scientific Corporation, CathRx Ltd, Biotronik SE & Co. KG., Japan Lifeline Co, ATRICURE, INC., Auris Health, and Biomerics.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/25474
What Else is in the Report?
Persistence Market Research offers a unique perspective and actionable insights on electrophysiology ablation market in its latest study, presenting historical demand assessment of 2016 – 2020 and projections for 2021–2031, based on product (diagnostic catheters, ablation catheters, and access devices), application (supraventricular tachycardia (SVT) ablation, ventricular tachycardia(VT/VPC), and atrial fibrillation (AF)), and end user (hospitals, ambulatory surgical centers, and clinics), across seven key regions of the world.
About us:
PersistenceMarketResearch is an esteemed company with a reputation of serving clients across domains of information technology (IT), healthcare, and chemicals. Our analysts undertake painstaking primary and secondary research to provide a seamless report with a 360 degree perspective. Data is compared against rep/uted organizations, trustworthy databases, and international surveys for producing impeccable reports backed with graphical and statistical information.
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
No comments:
Post a Comment