Non-invasive Prenatal Testing (NIPT) Market 2022
Advancing the prenatal care services provided across the globe has become an uphill battle for medical practitioners and drugmakers. Fortunately, this struggle is being eased by active participation of parents from all around the world in protecting the health of their soon-to-be-born babies through prenatal disease detection methods.
Get Free Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/11218
Noninvasive prenatal testing (NIPT) procedures are, hence, being rapidly adopted, which is prompting healthcare service providers to increase their focus on such prenatal diagnosis & screening services. Persistence Market Research recently published its study on the global market for noninvasive prenatal testing, which projects the market to bring in US$ 1,447.2 Mn revenues by the end of 2024.
At present, Persistence Market Research values the global noninvasive prenatal testing market at just over US$ 665 Mn, and expects the market to incur revenue growth at an impressive 10.2% CAGR. According to the report, titled “Noninvasive Prenatal Testing Market: Global Industry Analysis and Forecast, 2016-2027,” the global demand for noninvasive prenatal testing will keep surging on the account of rising innovations in prenatal screening products.
The growing presence of ADHD disorders among young adults is also triggering the adoption of noninvasive prenatal testing. Moreover, the demand for noninvasive prenatal tests is expected to grow as a majority of young adults are being introduced to generic medications. Nevertheless, stringent usage regulations and records of drug abuse are expected to curb the growing adoption of noninvasive prenatal testing to some extent.
Company Profiles:
- Ariosa Diagnostics, Inc.
- Berry Genomics Co. Ltd.
- BGI Diagnostics
- Illumina, Inc.
- LifeCodexx AG
- Natera, Inc.
- Sequenom Laboratories
- Quest Diagnostics, Inc.
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/11218
A majority of these companies are based in the US, which is why North America is projected to procure an average 60% of global NIPT revenues through 2024. The demand for noninvasive prenatal testing will also be more in Asia-Pacific, and the region’s NIPT market is looking at an opportune US$ 18 Mn increment in 2017 over 2016. Europe’s noninvasive prenatal testing revenues, on the other hand, will also grow aggressively – registering a 10% CAGR during the forecast period.
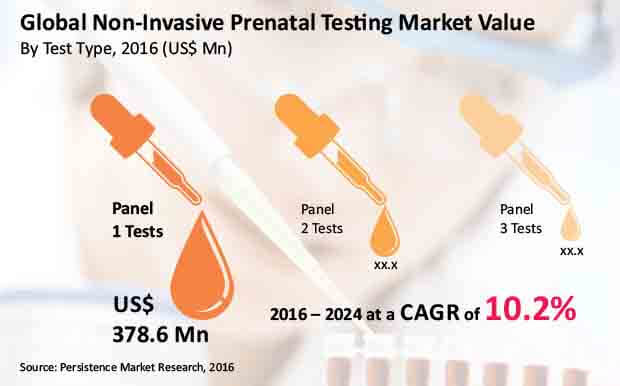
The report reveals that a considerable share of global NIPT revenues will be derived from higher preference to panel 1 test type. Adoption of panel 1 noninvasive prenatal testing has been projected to rise, bringing in over US$ 850 Mn revenues by the end of 2024. On the hand, global revenues from panel 2 tests are anticipated to soar at a healthy CAGR of 9.7%. Correspondingly, hospitals will remain the largest end-users of noninvasive prenatal testing throughout the forecast period. In the due course of this period, hospitals will not only account for half of global NIPT market value, but will also exhibit revenue growth at highest CAGR of 11.1%.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/11218
Global Noninvasive Prenatal Testing Market – Key Trends
- Impact of changing lifestyles: Urban livelihood and rising per capita income of individuals has propelled the occurrence of high-risk pregnancies. Unhealthy habits such as smoking, drinking and working around the clock has given rise to complications in maternal and neonatal outcomes.
- Advanced maternal age: Occurrence of women in their late 30s giving birth for the first time has increased. To avoid birth defects and consider the advanced age of mother, doctors are also promoting the adoption of noninvasive prenatal screening.
- Classification as “LDT”: More healthcare & pharmaceutical companies are entering the global NIPT market as these tests have been tagged as “lab-developed tests” by the FDA.
About Us: Persistence Market Research
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
No comments:
Post a Comment