Systemic Scleroderma Treatment Market 2022
In 2021, the systemic scleroderma treatment market was valued at nearly US$ 749.4 Mn, and is estimated to expand at a CAGR of 8% over the forecast period (2022-2032). Market growth is majorly attributed to the presence of innovative small molecule therapies, increasing clinical trials for concerned diseases, and significant government-backed funding. Furthermore, existing industrial and academic collaborations in developed countries are propelling systemic scleroderma treatment market growth.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/33054
The treatment for systemic scleroderma has been restricted to the off-label use of generic agents from other indications. However, recently, there has been a major shift in the market for this disease. Many researchers and sponsors are developing novel drugs for the treatment of scleroderma.
- In 2019, in the U.S. and Japan, Boehringer Ingelheim’s Ofev (nintedanib) was launched at a global level.
- In 2020, the European Commission approved nintedanib as a first therapy for the treatment of progressive systemic scleroderma-associated interstitial lung disease (SSc-ILD).
- In 2021, Roche/Genentech’s Actemra/RoActemra (tocilizumab) was approved in the U.S. for further expansion in the treatment of this disease.
Launching and approvals of novel drugs and therapies in recent years are positively impacting the systemic scleroderma treatment market, with more drugs in the pipeline and upcoming launching stages.
Company Profiles:
- Pfizer Inc.
- Novartis AG
- GlaxoSmithKline plc.
- Sanofi SA
- Lupin Ltd
- Cipla Ltd
- Teva Pharmaceuticals
- Johnson & Johnson Services Inc.
- Bayer Healthcare LLC
- F. Hoffmann-La Roche Ltd
- Amgen Inc
- Zydus Lifesciences Ltd
- Casper Pharma
- Boehringer Ingelheim Pharmaceuticals, Inc
- Organon LLC
- Accord Healthcare Inc
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/33054
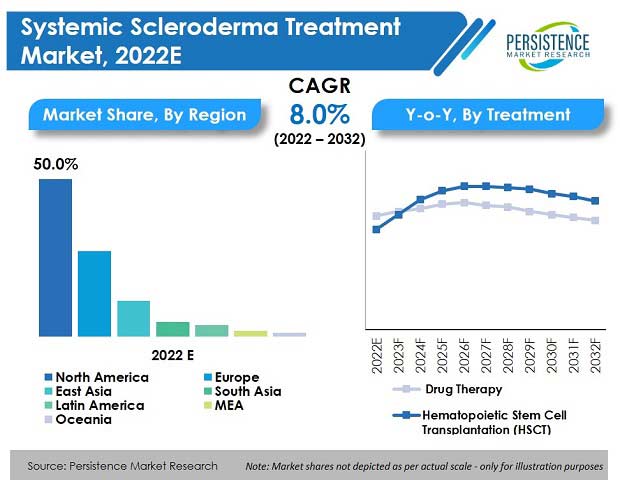
Key Takeaways from Market Study
- By treatment type, drug therapy is expected to hold over 95% market share by the end of 2032.
- The skin fibrosis segment is leading with nearly one-fourth market share in 2022.
- Injectables are expected to hold the largest share of 47.7% in 2022 amongst other routes of administration.
- Hospital pharmacies will dominate the global market with a value share of 41.4% in 2022.
- By region, North America is forecasted to be the leading regional market with a share of 51.8% by the end of 2032.
“Rising prevalence of systemic scleroderma, increasing R&D activities, and launch of novel therapeutics systemic scleroderma treatment procedures are favouring market expansion,” says an analyst of Persistence Market Research.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/33054
Market Competition
New product launches and product approvals are taking place with an appeal in untapped areas. This growth key strategy will create more demand and drive the systemic scleroderma treatment market.
- In March 2021, Janssen Pharmaceutical, a company of Johnson & Johnson, received approval from the U.S. Food and Drug Administration (FDA) for PONVORY (ponesimod) for the treatment of multiple sclerosis.
- In June 2021, Sanofi received approval from the European Commission for Aubagio (teriflunomide) for the treatment of relapsing-remitting multiple sclerosis.
- In March 2021, the U.S. FDA approved Genentech’s Actemra (tocilizumab) subcutaneous injection for the management of systemic sclerosis-associated interstitial lung disease.
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the systemic scleroderma treatment market in its latest study, presenting a historical demand assessment of 2017 – 2021 and projections for 2022 – 2032.
The research study is based on the target organ (skin fibrosis, musculoskeletal, digital ulcers, pulmonary arterial hypertension, gastrointestinal, pulmonary fibrosis and scleroderma renal crisis), treatment type (drug therapy(corticosteroids, immune-suppressants, nonsteroidal anti-inflammatory drugs, calcium channel blockers, proton pump inhibitors, TNF inhibitors, endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, angiotensin-converting enzyme) and hematopoietic stem cell transplantation (HSCT)), route of administration (injectable, oral and topical), and distribution channel (hospital pharmacies, specialty clinics, retail pharmacies and online pharmacies), across seven key regions of the world.
About Us: Persistence Market Research
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
No comments:
Post a Comment