North America Quadriplegia Care Devices Market 2022
The North America Quadriplegia Care Devices Market is destined to grow on an impertinent note, i.e. reaching US$ 741 Mn at a CAGR of 5.4% between 2021-2031. Personalization has taken over almost every sphere of the industry verticals, and healthcare is no exception. People have started looking at their healthcare picture such that they could actively take part in improving their physical and mental health on the daily basis. This personalization will be the face of the healthcare vertical in the forecast period.
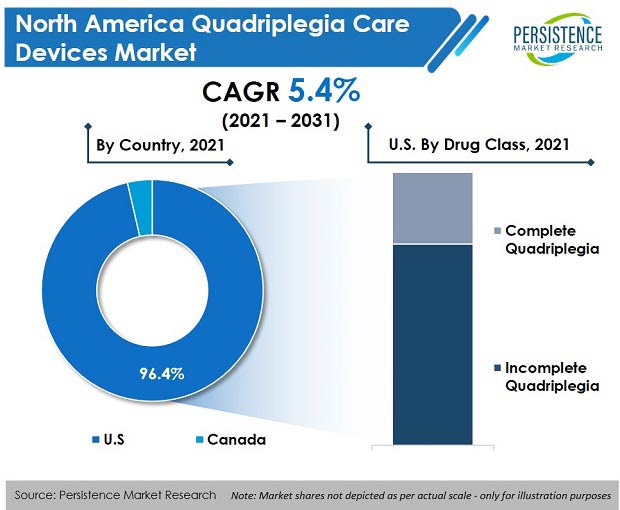
As per Persistence Market Research’s latest industry analysis, the North America quadriplegia care devices market recorded sales worth US$ 414.5 Mn in the year 2020, and is set to expand at a CAGR of 5.4% over the forecast period (2021-2031).
Increasing cases of sport-related injuries, including football, wrestling, gymnastics, diving, and surfing, have led to physical disabilities, and are positively impacting demand for quadriplegia care devices.
- Every year, more than 3.5 million sports injuries occur during some type of sporting event, according to the John Hopkins Medical Center.
After car accidents, falls are the second-most common cause of quadriplegia due to spinal cord injuries. It can also occur as a result of strain on the spinal cord in people with a history of malignancy.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/33058
Technology has introduced a new paradigm in devices that are connected to a patient’s body pressure or mobility-paralyzed patient. Artificial Intelligence has helped physically-disabled patients do their work independently. This emergence and acceptance of new technology by hospitals and paralyzed patients is expected to drive market expansion.
FES bikes are supercomputer products that have low-level electrical pulses that are transmitted through surface electrodes to the leg muscles for momentum.
- MyoCycle Home is an inexpensive, easy-to-operate FES bike that allows people with muscle weakness or paralysis to get the best workout possible, without leaving home.
FES systems are used for on-demand control of the paralyzed bladder and bowel. This emerging trend with functional electrical stimulation technology devices such as FES bikes, FreeHand, and Parastep will drive the growth of the quadriplegia care devices market.
Company Profiles:
- ReWalk Robotics
- Ekso Bionics
- Indego (Parker Hannifin Corporation)
- Rex Bionics Ltd.
- Caltech
- Össur
- Steeper, Inc.
- Boston Scientific corporation
- Medtronic Plc.
- Ottobock
- RGK Wheelchairs
- Rifton Equipment
- Allard USA, Inc.
- Fillauer LLC
- College Park Industries
- Mobius Bionics.
- Parker Hannifin
- Nevro
- ProBed Medical Technologies Inc.
- BLUE CHIP MEDICAL
- Restorative Therapies
- MYOLYN, LLC
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/33058
Key Takeaways from Market Study?
- Hospital beds accounted for the largest share of 27.7% in 2020 by product segment, as they are designed for patients to get continuous medical care and comfort.
- Incomplete quadriplegia occurs due to a vehicular accident or a sports injury. Since these situations are commonly observed across the world, incomplete quadriplegia held 73.8% market share by value in the year 2020.
- Demand for long-term care services for quadriplegia patients has become a major concern. The objective of these centers is to provide improved care, personalized attention, and neurological recovery for patients suffering from a spinal cord injury, which is why long-term care services accounted for the largest share of 38.9% by end user in 2020.
- The North America quadriplegia care devices market expanded at a CAGR of 5.4% over the forecast period (2021-2031). The market is expected to grow 1.7X by the end of 2031.
“Increasing incidence of spinal injuries due to road accidents and sports injuries will boost demand for quadriplegia care devices in North America over the years,” says an analyst of Persistence Market Research.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/33058
Market Competitions
Key manufacturers are investing in innovative, cost-effective, and user-friendly quadriplegia care devices to gain the attention of healthcare providers.
Additionally, companies are also aiming for various collaborations to create goodwill and successfully market their products.
- In March 2020, Allard USA, Inc. launched a new product – HUMERUX FRACTURE ORTHOSIS Y-traction system – that uses a traction device to increase and adjust the tension, helping maximize control over the alignment, fixation, and immobilization of the humerus.
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the North America quadriplegia care devices market in its latest study, presenting historical demand assessment of 2016 – 2020 and projections for 2021 – 2031.
The research study is based on the product (stimulation devices [spinal cord stimulator, functional electrical stimulation systems] assistive devices [exoskeletons, robotic arm system, prosthetics] mobility devices [wheelchairs, crutches and walkers, gait trainers] hospital beds, transfer bench and boards, braces & supports, orthopaedic splints, others supportive devices), based on indication (incomplete quadriplegia, complete quadriplegia) based on end-user (rehabilitation centres, assistive living facilities, long-term care centres, home care settings), across the continent.
About us: Persistence Market Research
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
No comments:
Post a Comment