Pulmonary Fibrosis Treatment Market 2022
The primary purpose of the statistical analysis is to use data analysis and descriptive statistics. It helps to summarize data from a sample using indexes like mean or standard deviation and inferential statistics. This report includes information and statistics on price levels, location of the product, and demand for the product. The best thing about the statistical analysis report is that it allows businesses to make decisions in terms of consumer preferences and purchasing power. Moreover, it provides demographic information like the number of potential customers in a geographical area, income level, consumer preferences, and much more. With the help of Pulmonary Fibrosis Treatment Market statistical analysis, the market could get crucial information on how the collected data and samples will be analyzed.
Monotherapy is cost-effective and gives deliverable action directly to the site of action in a few minutes, and is said to be a safe and effective method for treating idiopathic pulmonary fibrosis (IPF). According to Persistence Market Research (PMR), request for pulmonary fibrosis treatment is predicted to increase, enabling the market to surpass US$ 2.7 Bn by 2021. Over the coming years, rising cases of IPF will support expansion of the market.
Get Free Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/30070
High number of research & development projects, increasing investments, and favorable regulatory policies are some other factors supporting growth of the market for pulmonary fibrosis treatment. Majority of the population living with pulmonary fibrosis is being treated with traditional corticosteroid combination drugs with limited efficacy. With the arrival of new novel therapies, unaddressed populations in middle- and lower-income countries, as well as developed countries, can offer significant revenue generation opportunities.
Company Profiles:
- F. Hoffman – La Roche Ltd.
- Boehringer Ingelheim International GmbH
- Cipla Ltd (Cipla)
- Gilead Sciences, Inc.
- Bristol-Myers Squibb Company
- FibroGen Inc.
- Galecto Inc
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/30070
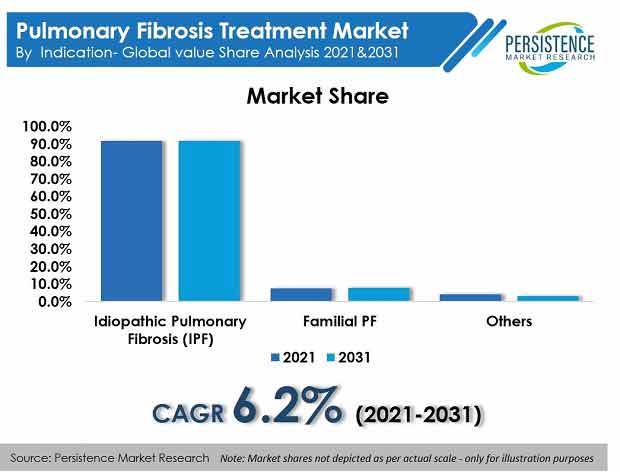
Key Takeaways from Market Study
- The global pulmonary fibrosis treatment market is expected to rise at a healthy CAGR over more than 6% through 2031.
- Increasing focus on effective treatment in the U.S is lending high Y-o-Y growth to the market in the country.
- Germany and France are expected to exhibit increasing demand to tackle concerns pertaining to rising incidence of IPF.
- Overall, the global market is set to expand 2X over the next ten years.
- Monotherapy to account for over 70% market share by 2031.
“Rising prevalence of idiopathic pulmonary fibrosis and importance of early diagnosis are creating growth opportunities for market players in the long term,” says a Persistence Market Research analyst.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/30070
Collaborations and Product Approval – Imperative Strategy for Market Players
Leading market players are strengthening their market position through collaborations with various other organizations. Global leading companies are focusing on research activities and approvals for increased market penetration.
- Roche, in March 2020, received Breakthrough Therapy Designation (BTD) from the U.S FDA for Esbriet (pirfenidone), for use in adults with unclassifiable interstitial lung disease (uILD).
- In June 2019, Boehringer Ingelheim entered into a collaboration and license agreement with Bridge Biotherapeutics Inc. (South Korea), with the aim to fast-track the development of Bridge’s auto tax in inhibitor BBT-877 for treatment against fibrosing interstitial lung diseases, including idiopathic pulmonary fibrosis, a rare lung disease.
- In October 2020, Cipla introduced the generic version Nintedanib for the treatment of idiopathic pulmonary fibrosis. In India, this drug will be marketed under the brand name Ninitb. Nintedanib will be available in two formulations of 100 mg and 150 mg.
What else is in the report?
Persistence Market Research offers a unique perspective and actionable insights on the pulmonary fibrosis treatment market in its latest study, presenting historical demand assessment of 2016 – 2020 and projections for 2021 – 2031, based on therapy (monotherapy, combination therapy and symptomatic treatment), indication (idiopathic pulmonary fibrosis (IPF), familial PF, and others), and distribution channel (hospital pharmacies, retail pharmacies, and mail order pharmacies), across seven key regions of the world.
About Us: Persistence Market Research
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
No comments:
Post a Comment